AviClear uses proprietary technology to deliver safe and effective acne laser treatments with no need for adjunctive pain therapy
Avi360™ partnership offers a suite of tools and services for easy integration into dermatology practices
CUTERA, INC. (Nasdaq: CUTR) ("Cutera" or the "Company"), a leading provider of dermatology solutions, today announced that the first patient outside of clinical trials was treated with AviClear, the only device cleared by the FDA for the treatment of mild, moderate, and severe acne.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220405005446/en/
(Graphic: Business Wire)
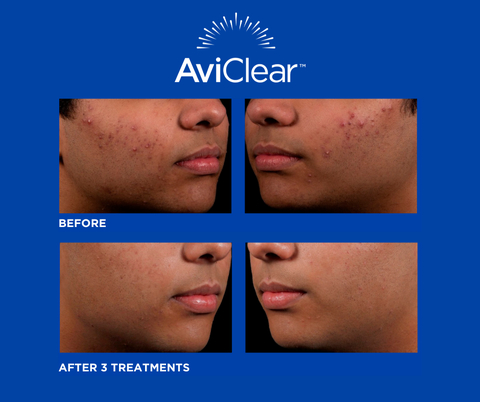
AviClear received FDA 510k clearance on March 24, 2022, following the Company’s successful product development process and extensive clinical evaluation. Designed with physician and patient needs in mind, AviClear significantly reduces the severity and frequency of acne following three brief sessions without any adjunctive pain therapy. AviClear rapidly delivers laser energy in combination with AviCool™, a unique handpiece feature that provides unmatched contact cooling capability, delivering a more comfortable treatment experience.
“The patient I treated with the AviClear device has suffered from moderate to severe acne for the past several years and has been seeking a safe, effective, and drug-free treatment option,” said Julie Harper, M.D., Past President of the American Acne and Rosacea Society. “In light of the recent FDA clearance, I advised her that this new treatment option involved no downtime and has had no adverse events throughout its extensive trialing. The AviCool feature allows me to deliver a highly effective treatment without any anesthetic. All of these benefits made my patient very excited to try it.”
"Now that AviClear treatments have begun, we look forward to partnering with physicians to redefine the acne armamentarium,” said David Mowry, CEO of Cutera. “We are completely focused on eliminating the complexity and administrative burdens associated with current acne treatment options. To deliver on that commitment, we are proud to provide Avi360, a comprehensive practice support program that makes it easier for dermatologists to acquire the system and market it effectively to their patients. From a clinical standpoint, our novel and differentiated approach offers a safer, simpler, and effective solution to this chronic skin condition.”
AviClear is rolling out to physicians throughout the United States over the course of 2022. Doctors and patients are encouraged to visit www.AviClear.com to sign up for updates on product availability and local treatment providers.
About Cutera, Inc.
Brisbane, California-based Cutera is a leading laser and other energy-based systems provider for dermatologists and aesthetic practitioners worldwide. Since 1998, Cutera has been developing innovative, easy-to-use products that harness the power of science and nature to enable physicians and other qualified practitioners to offer safe and effective treatments to their patients. For more information, call +1 415-657-5500 or 1-888-4CUTERA or visit www.cutera.com.
References:
- White paper on cooling capacity of various laser skin treatment options, Data on file.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220405005446/en/
Contacts
EvolveMKD
Bridget Callahan
Cutera@EvolveMKD.com





