Cook Medical issued a global, voluntary recall of the Transseptal Needle and the Transseptal Needle with Catheter. This recall includes all unexpired lots for both of these products. The needles were recalled due to complaints of rust on the products. Use of affected products could result in increased procedural time and inflammatory reactions, including systemic reactions which may lead to permanent impairment or death.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211018006006/en/
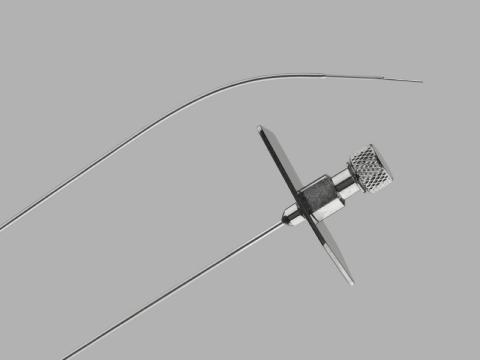
Cook Medical's Transseptal Needle product (Photo: Business Wire)
The U.S. Food and Drug Administration (FDA) has not yet classified the recall. A complete list of products affected by this recall can be found below.
Transseptal Needles, including Transseptal Needles with Catheters, were found to have rust internally, externally, or both. To date, Cook Medical has received no reports of injury or illness related to this recall. Cook has received four complaints where the presence of rust was identified prior to patient contact. However, please be advised that the presence of rust may go undetected by the user. The FDA and other regulatory agencies around the world have been notified of this action.
Potential adverse events
If an affected product is used, potential negative outcomes include increased procedural time (to obtain a replacement device) and inflammatory reactions ranging from local or self-limited reactions to systemic reactions requiring medical intervention. Systemic reactions could potentially lead to permanent impairment or death.
Affected products
PRODUCT BRAND NAME |
INTENDED USE |
REFERENCE PART
|
ORDER NUMBER (GPN) |
LOT NUMBER / UDI |
RANGE OF
|
Transseptal Needle |
Intended for transseptal
|
TSNC-18-71.0 |
G02364 |
|
|
TSNC-19-56.0 |
G02365 |
All | October 02, 2016 through July 22, 2021 |
||
Transseptal Needle with Catheter |
Intended to facilitate transseptal
|
TSN-17-75.0-ENDRYS |
G19261 |
Recall return information
Customers that received products affected by the recall were sent prepaid labels in a recall information packet. The letters requested that all customers and distributors quarantine and discontinue use of all potentially affected units and return the affected products to Cook as soon as possible for credit.
Customers in the U.S. and in Latin America should return affected product(s) to Cook Medical. Before returning affected product to Cook Medical, please obtain an RGA#. You may contact 800.457.4500, press option 5, and enter extension 151090 to obtain an RGA# and return address.
Adverse event reporting
Report adverse events to Cook Medical Customer Relations. Adverse events or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
Cook Medical Customer Service
Phone: 1.800.457.4500 or 1.812.339.2235, Monday – Friday between 7:30 am – 5:00 pm (EST)
Email: CustomerRelationsNA@cookmedical.com
U.S. Food and Drug Administration
Phone: 1.800.FDA.1088
Web: http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm
FDA MedWatch Adverse Event Reporting
Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211018006006/en/
Contacts
Hannah Chudleigh
External Corporate Communications, Cook Medical
+1.812.345.5943
hannah.chudleigh@cookmedical.com